Abstract
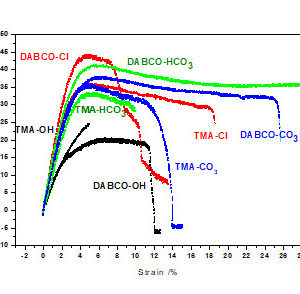
The thermomechanical properties of anion exchange polymers based on polysulfone (PSU) quaternized with trimethylamine (TMA) or 1,4-diazabicyclo[2.2.2]octane (DABCO) and containing hydroxide or chloride anions by tensile stress-strain tests and dynamic mechanical analysis (DMA) have been determined. The reported mechanical properties included the Young’s modulus, tensile strength, and elongation at break from tensile tests and the storage and loss modulus and glass transition temperature from DMA. The anion exchange membranes behaved as stiff polymers with Young’s modulus in the order of 1 GPa, relatively with high strength (about 30 MPa) and low elongation at break (around 10%) was observed. Tensile tests were also made with membranes exchanged with hydrogen-carbonate and carbonate anions to control the absence of important carbonation of the OH form. The glass transition temperatures were of the order of 150 °C (PSU-TMA) or 200 °C (PSU-DABCO) for the hydroxide form, confirmed by differential scanning calorimetry; they increase further by about 50 K, when hydroxide ions are replaced by chloride. This result and the increase of the storage modulus could be interpreted by the higher hydration of hydroxide ions and the plasticizing effect of water, which reduced the Van der Waals interactions between the macromolecular chains.
Illustrations
Details
Published on: Journal of Polymer Science Part B: Polymer Physics 2016, 54, 1180–1187
Authors: R. Narducci, J.‐F. Chailan, A. Fahs, L. Pasquini, M. L. Di Vona, P. Knauth