Ion conducting polymers are typically phase-separated into a percolating network of hydrophilic nanopores embedded in a hydrophobic polymer-rich phase domain. The hydrophilic nanopores contain the charged moieties that ensure ion conductivity; the hydrophobic phase domain provides mechanical strength.
The research in the LIME Lab is focused on obtaining ion exchange membranes with high chemical, hydrolytic, and thermal stability, good mechanical properties, and high conductivity. To obtain all the desired qualities, materials with a complex architecture consisting of a hydrophobic rigid main polymer chain that guarantees mechanical and chemical resistance combined with hydrophilic groups responsible for ion conduction must be elaborated.
Proton exchange membranes
We have studied two different classes of ionomers: sulfonated aromatic and perfluorinated polymers. Sulfonated aromatic polymers (SAPs) are promising alternative membrane materials. The most important examples are the poly(ether ether ketone) (PEEK) and poly(phenyl sulfone) (PPSU) polymer families, which are very well-known high temperature engineering resins. Cross-linked SAPs were prepared during the European Project LoLiPEM by solvothermal macromolecular synthesis. This elegant method increases spectacularly the mechanical and hydrolytic stability of sulfonated aromatic polymers, making them suitable for use in liquid water also at 145°C. Fuel cell tests are very promising. The cross-linked membranes were also used for air to air enthalpy exchangers (Patent). The formation of water insoluble hybrid materials enabled the preparation of membranes with good thermal stability and reduced swelling, whose conductivity seems to be promising for fuel cell applications. The introduction of Si(OH)3 units gives the possibility of further improving the hybrid characteristics by soft chemical methods, such as sol/gel processes. Composites with an interpenetrating network or oxide nanoparticles were synthetized and tested.
For perfluorinated ionomers (PFSA), we investigated the possibility to increase the working temperature and endurance of proton exchange membranes by thermal annealing of short and long side chain perfluorosulfonic acid Aquivion and Nafion. The Ionomer nc Analysis (INCA method), based on nc/T plots, where nc is a counter elastic force index, was applied to evaluate the ionomer thermo-mechanical properties and to probe the increase of crystallinity during the annealing procedure.
Anion exchange membranes
Anion exchange membranes (AEM) are very versatile ion conducting materials used in several applications and many fields can benefit from their application: in alkaline fuel cells, they avoid the extensive use of platinum or related expensive noble-metal electrocatalysts for the oxygen reduction reaction, and reduce problems related to carbonation. AEM are formed by a polymeric backbone functionalized with fixed cationic groups and counter anions, generally hydroxide ions that sustain the conductivity. The mediocre alkaline stability is the main problem for the widespread use of anion exchange membranes in alkaline fuel cells and other electrochemical devices.
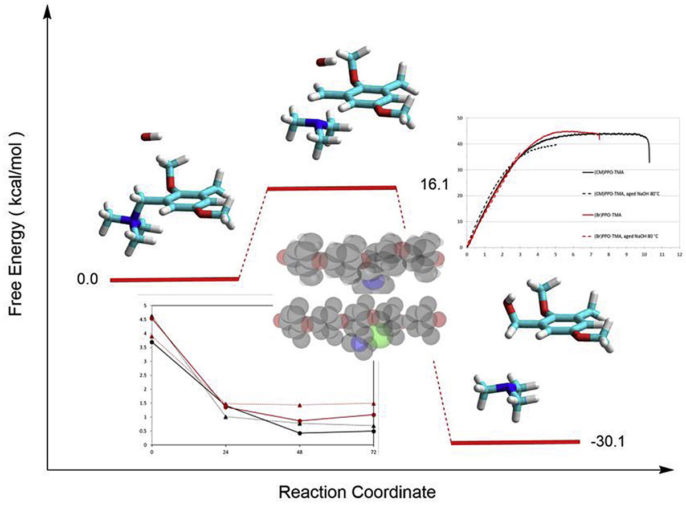
Fundamental studies were conducted to ascertain how the use of different anion exchange groups and the crosslinking of the matrix can influence the properties of the material. The degradation in alkaline environment of different backbones was studied by various analysis techniques. The effect of the matrix structure on the alkaline stability of model anion exchange polymers based on PPO with grafted trimethylammonium groups showed contrasting results. From the point of view of the mechanical properties, the samples with pendent methyl groups revealed the best results: the polymer was more ductile also after the aging treatment. Concerning the hydroxide ion concentration dependence of the degradation, the kinetic analysis must differentiate the rate of a reaction with the internal OH ions, which are in equal concentration with the grafted ammonium counter-ions, from the treatment inside an external NaOH solution. In the latter case, the OH concentration, in large excess in the external solution, can be considered constant and the reaction rate becomes pseudo-first order. The stability in alkaline condition of long-chain PPO membranes synthetized by a metalation route, studied by thermogravimetry, ion conductivity, and IEC measurements, is improved versus short side-chain ionomers. The alkaline degradation is rapid during the first hours of the reaction and shows a slower kinetics at longer times.
The conduction of different anions was measured and in the case of fluoride ions the diffusion coefficients obtained by NMR and conductivity measurements were compared. The formation of composites stabilizes the membranes, especially when it is possible to obtain homogeneous materials.
.