Abstract
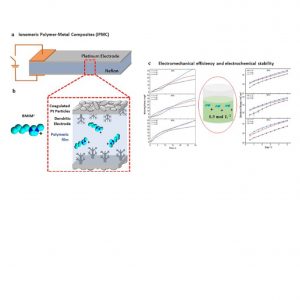
In the field of soft actuators, Ionomeric Polymer Metal Composites (IPMC)-like devices are a trend, exhibiting large displacement with low applied voltage. Its working mechanism is related to solvated electrolytes migration, thus the number of counterions exchanged with the polymeric membrane plays a key role in the device’s performance. Although many kinds of inorganic and organic ions were used, there were few efforts to address a specific concentration value of electrolyte solutions. Ionic liquids (ILs) are used in IPMC to provide electrochemical stability; however, their mechanical performance is usually poor. In this study we aimed to determine a specific value of 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) ionic liquid concentration between 0.1, 0.3, and 0.5 mol L-1 that grants electrochemical stability at different relative humidities with best electromechanical efficiency. We synthesized [BMIM]Cl and characterized it through Nuclear Magnetic Resonance (NMR), Fourier Transform Infrared Spectroscopy (FTIR), and Cyclic Voltammetry (CV). The electrochemical behavior of Nafion®/Pt-based IPMC exchanged with IL was studied through Electrochemical Impedance Spectroscopy (EIS), CV, and Chronoamperometry (CA). Electromechanical properties were measured through blocking force and displacement. All the IPMC tests were carried out at three distinct controlled humidities (30%, 60%, and 90%). Herein, we tuned the IL concentration in 0.3 mol L-1, delivering electrochemical stability with the best electromechanical yield regardless of the relative humidity. This result will be important when bringing electrolyte mixtures to further enhance the performance and efficiency of these devices.
Illustrations
Details
Published on: Journal of Applied Electrochemistry 2023, 53(2), 241-255
Authors: K.A. Tozzi, R. Gonçalves, R. Barbosa, M.C. Saccardo, A. Zuquello, E. Sgreccia, R. Narducci, C.H. Scuracchio, M.L. Di Vona