Abstract
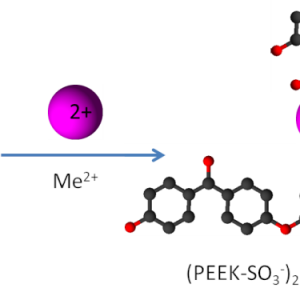
Ion exchange polymers were used for mercury and lead ions removal in water. The heavy metal ion concentration was analyzed by two independent methods: inductively coupled plasma–optical emission spectroscopy (ICP-OES) and gravimetry. The studied cation exchange polymer (CEP) was sulfonated poly(ether ether ketone) (SPEEK), and the anion exchange polymer (AEP) was poly(sulfone trimethylammonium) chloride (PSU-TMA). The removal capacity was connected with the ion exchange capacity (IEC) equal to 1.6 meq/g for both polymers. The concentration ranges were 0.15–0.006 mM for Hg2+ and 10.8–1.0 mM for Pb2+. SPEEK achieved 100% removal efficiency for mercury and lead if the concentration was below the maximum sorption capacity (Qmax), which was about 210 mg/g for Pb2+ with SPEEK. For PSU-TMA, the surprising removal efficiency of 100% for Hg2+, which seemed incompatible with ion exchange, was related to the formation of very stable complex anions that can be sorbed by an AEP. Langmuir adsorption theory was applied for the thermodynamic description of lead removal by SPEEK. A second-order law was effective to describe the kinetics of the process.
Illustrations
Details
Published on: J. Materials Science, 2024, 59, 2776-2787.
Authors: Sgreccia E.; Rogalska C.; Gallardo Gonzalez F.S.; Prosposito P.; Burratti L.; Knauth P.; Di Vona M.L.