Abstract
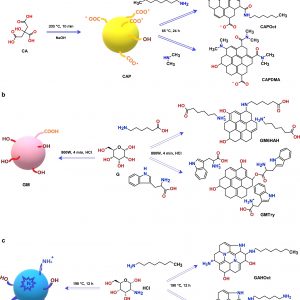
Carbon quantum dots (CQD) were prepared from three different precursors and by three bottom-up synthesis methods: classical pyrolysis of citric acid (CAP), microwave irradiation of glucose (GM), and hydrothermal treatment of glucosamine hydrochloride (GAH). CQD were further functionalized using various nitrogen-containing compounds: 6-aminohexanoic acid, 1,6-diaminohexane, N-octylamine, dimethylamine, and tryptophan. Special attention was dedicated to investigate how the combination of synthetic method and starting material affected the nature and properties of CQD. The analysis indicated that CAP were good candidates for covalent post-functionalization, GM allowed an easy passivation, and GAH permitted the direct introduction of nitrogen into the core. The size distribution showed a core–shell structure for CQD functionalized with an aminoacid by microwave irradiation, whereas the thermal decomposition evidenced the degradation of functionalizing molecules and the presence of pyridinic and pyrrolic nitrogen after hydrothermal synthesis. Photoluminescence spectra revealed important differences between the synthesis techniques, related to the occurrence of surface states, and the highest fluorescence quantum yield for hydrothermally prepared CQD. These approaches led to CQD with properties that can be exploited in many fields from energy conversion to sensing.
Illustrations
Details
Published on: Journal of Nanostructure in Chemistry (2021)
Authors: A. R. Nallayagari, E. Sgreccia, R. Pizzoferrato, M. Cabibbo, S. Kaciulis, E. Bolli, L. Pasquini, P. Knauth, M. L. Di Vona