Abstract
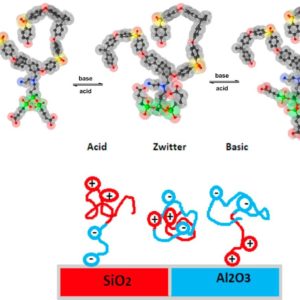
Stimuli-responsive hybrid organic-inorganic amphoteric ion exchange polymers were synthesized via a non-hydrolytic sol–gel process with carboxylic acid and amine groups in silica side chains. Structural characterization of samples with a ratio carboxylic acid/amine 1:2 (PSU-SiCA(1:2)) and 2:2 (PSU-SiCA(2:2)) showed a degree of functionalization of 0.5 in both cases. The ion exchange capacity, analysed by acid-base titration, elemental analysis and thermogravimetric analysis, was 1.8 meq/g for PSU-SiCA(1:2) and 1.2 meq/g for PSU-SiCA(2:2). pH-Dependent properties for acidic, basic and zwitterionic forms were studied including hydration isotherms, ionic conductivity and selective deposition on acidic and basic substrates. The water uptake and ionic conductivity of the acidic and basic forms were highest for PSU-SiCA(1:2), due to the higher IEC. The zwitterionic forms, which contain a minimum of mobile ions, showed the lowest water uptake and ionic conductivity. The acidic form deposited on basic Al2O3 substrates and the basic form on acidic SiO2, whereas the zwitterionic polymer was deposited on neither. The stimulus-dependent ionic conductivity and hydration and the selective deposition depending on interfacial charges are striking properties, which make these hybrid bio-friendly ionomers compelling for applications.
Illustrations
Details
Published on: European Polymer Journal 2019, 112, 255–262
Authors: E. Sgreccia, L. Pasquini, G. Ercolani, P. Knauth, M. L. Di Vona