Abstract
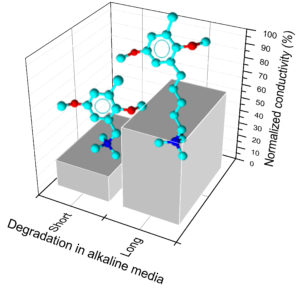
The stability of anion exchange membranes is paramount for the use in alkaline fuel cells. Long-chain ionomers are supposed to be more alkaline-resistant with respect to short-chain isomers. In this paper, the synthesis, properties, and stability of ionomers with a long side chain are investigated. Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO) is chosen as the backbone because of its reported stability in alkaline conditions. The functional group is pentyl-ammonium with trimethylamine (TMA) or 1,4-diazabicyclo[2.2.2]octane as model amines. The synthesis is carried out via metalation reaction and is optimized as a function of temperature and time. The water uptake is relatively low in accordance with the large hydrophobicity of the PPO backbone. The through-plane ionic conductivity is consistent with literature data; it amounts to 15.3 mS/cm at 80 °C for the TMA derivative. The mechanical properties are typical of ionomers below the glass-transition temperature (for the TMA derivative at ambient humidity: Young modulus = 1310 ± 30 MPa). The stability in alkaline conditions, studied by thermogravimetric analysis and measurements of ionic conductivity and ion exchange capacity (IEC), is higher than that of short side-chain ionomers with the same basic group. The decrease of ionic conductivity (57 vs 22% residual conductivity after 72 h in 2 M NaOH at 80 °C) and IEC is monitored, showing that the degradation is fast in the first hours and may be described by second-order kinetics. These results help in selecting high-performance anion exchange membranes for electrochemical energy technologies.
Illustrations
Details
Published on: Journal of Physical Chemistry C 2020, 124, 1309-1316
Authors: R.-A. Becerra-Arciniegas, R. Narducci, G. Ercolani, E. Sgreccia, L. Pasquini, M. L. Di Vona, P. Knauth