Abstract
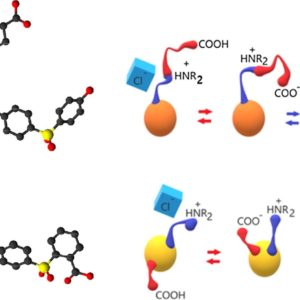
Amphoteric ionomers were synthesized with two different approaches to study the influence of a flexible side chain containing the functional groups vs grafting of the functional groups directly on the main chain. Poly(sulfone) was functionalized with 6-aminohexanoic acid (ANF-1) or by successive carboxylation and amination of the aromatic main chain (ANF-2). The samples were characterized by FTIR and NMR spectroscopies and thermogravimetric analysis. Acid and base constants were measured by titration. We also determined the hydration and the ionic conductivity for acid, base and zwitterionic forms made by variation of the pH. The basic forms containing dissociated carboxylic groups and mobile cations showed a higher water uptake and a higher conductivity. The zwitterionic forms presented a low water uptake for both polyelectrolytes and the lowest conductivity for ANF-2. The reduced conductivity in acidic conditions of ANF-1, where amine groups were protonated and only anions were mobile, was related to the position of ammonium moieties inside the side chain. This work showed how the properties of stimuli-responsive polymers depended on the position of the functional groups.
Illustrations
Details
Published on: European Polymer Journal 2019, 119, 45-51
Authors: R. Narducci, E. Sgreccia, G. Ercolani, M. Sette, S. Antonaroli, L. Pasquini, P. Knauth, M. L. Di Vona