Abstract
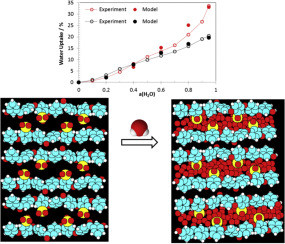
The hydration isotherms of various ionomers (Nafion, Sulfonated Poly-Ether-Ether-Ketone, Sulfonated Poly-Phenyl-Sulfone) were measured and described by a thermodynamic model, assuming ideality and a linear relation between the thermodynamic osmotic pressure and the volume of the internal electrolytic solution. The only two parameters used are related to measurable physical properties: the deformation parameter, inversely proportional to the elastic modulus of the ionomer, and the free volume parameter. The experimental trends are well reproduced by the model, showing that it captures the main physical features and is suitable for semi-quantitative analysis of ionomer hydration. It indicates that the ionomers behave in good approximation like elastic solids in the studied range of water activities (0 < a(H2O) < 0.95). The implications for practical improvement of ionomer properties by thermal cross-linking and annealing treatments are discussed. Cross-linked ionomers do not only better resist swelling at high humidity, but also dehydration at low humidity, which should increase their durability.
Illustrations
Details
Published on: Journal of Power Sources 2014, 267, 692-699
Authors: P. Knauth, E. Sgreccia, M. L. Di Vona