Abstract
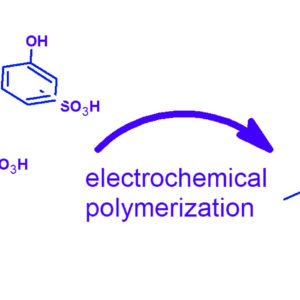
The electrochemical deposition of sulfonated poly(phenyl ether) (SPPE) from sulfonated phenol precursors was studied by cyclic voltammetry (CV) and chronoamperometry. A well-defined peak was observed by CV with a maximum current around E=1.35 V vs Ag/AgCl. The potentiostatic electropolymerisation at a potential of 1.3 V led to the formation of a homogeneous SPPE layer with a Faradic efficiency above 90 %. The ortho-isomer can be deposited with around 4 times higher current density than the para-isomer, due to the different steric hindrance. The chronoamperometric curves were used to analyse the mass-transport limited growth of SPPE. The diffusion coefficient of the precursor molecules in the SPPE layer can be determined from the square root time dependence of the current. The diffusion coefficient of the o-isomer is higher than that of the p-isomer (3 10−9 vs. 4 10−10 cm2s−1), pointing to the higher volume of the p-isomer reducing the diffusion rate in SPPE. The obtained polymers were characterized via Fourier-Transform Infrared, Nuclear Magnetic Resonance, and Electrochemical Impedance Spectroscopy. The ion conductivity of SPPE at 20 °C in fully humidified conditions attains (6 ± 2) mS.cm−1. The electrochemical coating of complicated electrode or electrocatalyst morphologies opens the way to 3D nanoarchitectures.
Illustrations
Details
Published on: ChemistrySelect 2016, 1, 3114-3119
Authors: M. Braglia, I. V. Ferrari, F. Vacandio, P. Knauth, M. L. Di Vona