Abstract
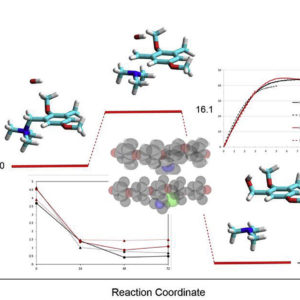
We study the effect of pendent methyl groups on the alkaline stability of model anion exchange polymers based on poly(2,6-dimethyl-1,4-phenylene)oxide (PPO) with grafted trimethylammonium groups. The polymer backbone is modified varying the synthesis procedure: by the bromination route, the ionomeric ammonium groups are inserted on the structural methyl group of PPO (Br-PPO-TMA), while by the chloromethylation route the ammonium groups are attached in the ortho-position to the methyl group of PPO (Cl-PPO-TMA). The properties of the membranes are studied by NMR and FTIR spectroscopy, thermogravimetry, water uptake, mechanical tensile tests, small-angle X-ray scattering (SAXS) and impedance spectroscopy. SAXS analysis indicates a better nanophase separation for Cl-PPO-TMA, which is consistent with a slightly larger water uptake and ionic conductivity. The properties of the two polymers are also compared before and after the aging in 2 M NaOH at 80 °C for different times. The thermogravimetric analysis shows the loss of the ammonium groups and the backbone ether cleavage after alkali treatments. The samples prepared by the bromination route show a higher stability of ionic conductivity, presumably due to a reduced alkaline attack of the ammonium groups, although DFT calculations do not show major differences of thermodynamic and kinetic reaction parameters. After alkaline treatment, the mechanical properties are more degraded for the Br-PPO-TMA compound. The decreased mechanical properties can be attributed to a reduction of the average chain length of PPO by alkaline scission of the ether links. Both synthesis routes have advantages and disadvantages, but the higher reproducibility and the better mechanical properties of samples prepared by chloromethylation are considered preponderant benefits.
Illustrations
Details
Published on: Polymer 2019, 185, 121931
Authors: R.-A. Becerra-Arciniegas, R. Narducci, G. Ercolani, S. Antonaroli, E. Sgreccia, L. Pasquini, P. Knauth, M.L. Di Vona