The Project
Brief description of the project
Fuel cells will have a crucial role in a more sustainable and green economy, to produce the energy by renewable sources and reduce greenhouse gas emissions.
Anion exchange membranes fuel cell (AEMFC) have significant potential advantages with respect to other FC technologies. However, a more sustainable FC, to obtain not only environmental benefits for the production of renewable energy, must move towards sustainable materials with a composition of abundant and easily accessible elements, and with production possibilities based on renewable raw materials.
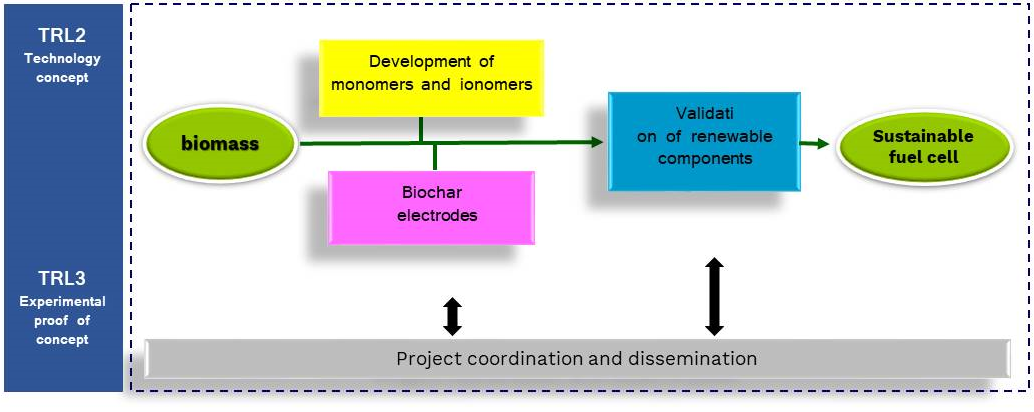
The main objective of GreenCore is to set-up a sound approach to the development of core components of eco-friendly AEMFC, exploiting biomass resources, employing green technologies, and finding alternative greener materials.
Objectives
Realizing the core components of an anionic exchange membrane fuel cell (membranes and electrodes) by exploiting biomass resources with innovative green chemistry.
The compliance with the green chemistry guidelines will ensure that the targeted products are genuinely sustainable since it implies optimizing processing and product design to avoid waste and inadvertent environmental damage, besides replacing unsustainable feedstocks.
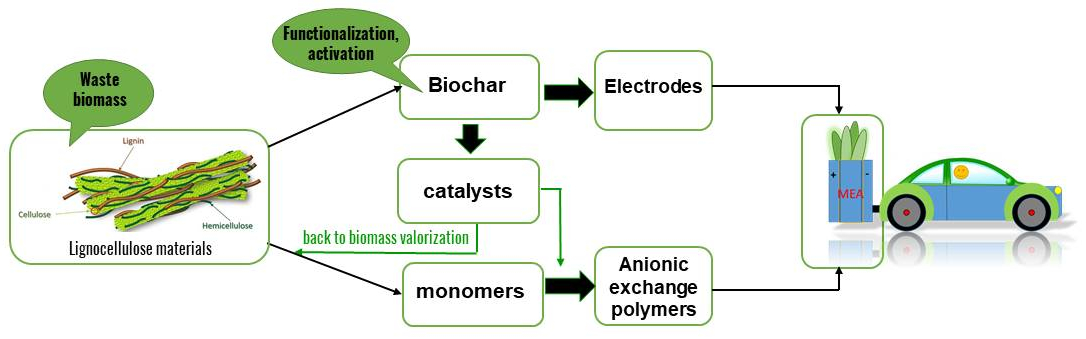
Environmental impact
GreenCore will fully address the principle of DNSH since it focuses on green and sustainable methodologies to hydrogen production. The climate change we have been facing in recent years has led to a growing awareness of man-made damage and its consequences for future generations. One of the main causes of the increase in global average temperatures is due to the greenhouse gas, which is mostly generated by the combustion of fossil energy carriers. The production and use of clean energy is then mandatory (Paris Agreement in 2015 and COP27 2022 Egypt). In agreement with Europe’s climate change mitigation targets, hydrogen has a strategic importance in the development of a low-emission, environmentally friendly, and more sustainable energy system.
Anionic exchange membrane fuel cells (AEMFC) seem very promising because they allow low or zero PGM levels. However, considering the current state-of-the-art and the low level of technological readiness (TRL) for AEMFC, there is significant potential to improve production emissions, as the development of the technology will lead to less material intensity and cleaner production routes. In this context, the GreenCore project can make a contribution linked to sustainability and environmental protection.
Scientific and technological impacts
Proof-of-concept of “fully green” fuel cell technology.
With this research project, we are promoting a symbiosis between a green energy source and green chemistry, that efficiently uses renewable raw materials, minimizing waste and avoiding the use of toxic and hazardous substances in the design-manufacturing-application of chemicals or materials.
Social impacts
- The Italian “Hydrogen Strategy” sets a medium and a long-term objective, according to which the national energy consumption is expected to consist of 2% hydrogen by 2030 and 20% by 2050. It can be assumed that the demand for FC will increase dramatically in the future, along with the need for CRM for their production. With this project, we are providing a contribution towards ensuring that FC can be manufactured in high volumes in a resource-efficient, green and cost-effective manner
- Improve public perception of the scientific and societal impact of new technologies by a coherent communication strategy of outcomes, and promoting green technologies-related education and training programmes
Workplan
Public documents
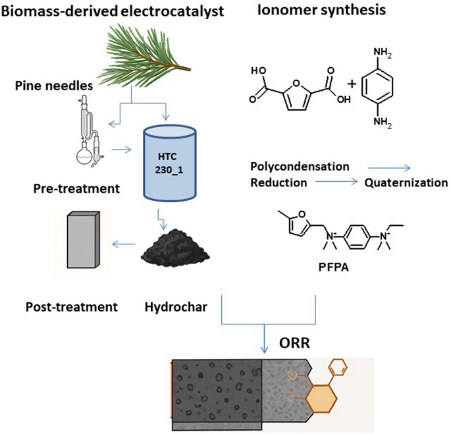
Sustainable electrodes based on biomass-derived catalysts and Ionomers for the Oxygen Reduction Reaction
Authors: S. Chandrasekaran, R. Narducci, E. Sgreccia, A. Haider, L. Pasquini, A. Marrocchi, E. Cerza, M. L. Di Vona and P. Knauth
Published on: Journal of Materials Science: Materials in Energy 2025, 1, 7
Abstract
Anion Exchange Membrane Fuel Cells (AEMFCs) are promising clean energy devices. To align their developmentwith sustainability goals, this study explores the integration of biomass-derived components into AEMFC cathodes.Biochar-based electrocatalysts were synthesized via hydrothermal carbonization (HTC) of pine needles, a water-based, low-temperature process. Three processing strategies were investigated: direct HTC of raw biomass,HTC following biomass pre-treatment, and thermal post-treatment of the resulting hydrochar to promotegraphitization. In parallel, a biomass-derived anion exchange ionomer (AEI) was synthesized via polycondensationof 2,5-furandicarboxylic acid with p-phenylenediamine, followed by reduction and quaternization. The resultingfuran-based AEI was incorporated into the cathode and benchmarked against a PPO-based ionomer (PPO-LC).Electrochemical testing showed that the furan-based AEI led to lower performance, particularly fewer exchangedelectrons, though onset potentials remained comparable to the Pt/C reference. Hydrochar derived from raw pineneedles demonstrated favourable performance with minimal processing, avoiding organic solvents and loweringthe energy demand. In contrast, a high temperature post-treatment offered only marginal performance gainsat high energy cost, limiting its sustainability. This study highlights the importance of balancing performance,processing effort, and environmental impact in AEMFC electrode development.
Read the full article on Springer
One-Component Catalytic Electrodes from Metal–Organic Frameworks Covalently Linked to an Anion Exchange Ionomer
Authors: R. Narducci, E. Sgreccia, A.V. Montella, G. Ercolani, S. Kaciulis, S. Syahputra, E. Bloch, L. Pasquini, P. Knauth, M.L. Di Vona
Published on: Molecules 2025, 30(6), 1230
Abstract
Anion-conducting organic–inorganic polymers (OIPs), constructed using metal–organic framework (MOF)-like structures with non-toxic, non-rare catalytic metals (Fe3+, Zr4+), have been developed. The incorporation of MOF-like structures imparts porosity to the polymers, classifying them as porous organic polymers (POPs). The combination between catalytic activity, ion conduction, and porosity allows the material to act as one-component catalytic electrodes. A high catalytic activity is expected since the entire surface area contributes to electrocatalysis, rather than being restricted to triple-phase boundaries. The synthesis involved anchoring a synthon onto a commercial polymer, assembling organo-metallic moieties, and functionalizing with quaternary ammonium (QA) groups. Two hybrid materials, Zr-POP-QA and Fe-POP-QA, were thoroughly characterized by NMR, FTIR, XPS, BET surface area (≈200 m2/g), and TGA. The resulting electrodes demonstrated a high electrochemically active surface area and a high efficiency for the oxygen reduction reaction (ORR), a critical process for energy storage and conversion technologies. The performance was characterized by a 4-electron reduction pathway, a high onset potential (≈0.9 V vs. RHE), and a low Tafel slope (≈0.06 V). We attribute this efficiency to the high active surface area, which results from the simultaneous presence of catalytic transition metal ions (Zr or Fe) and ion conducting groups.
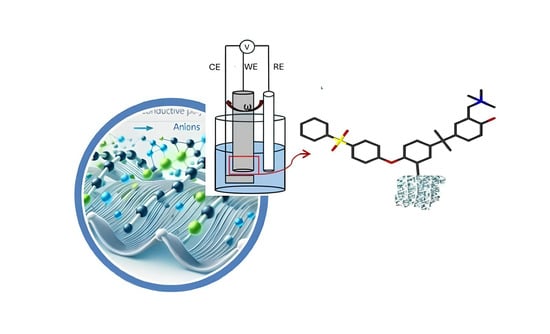
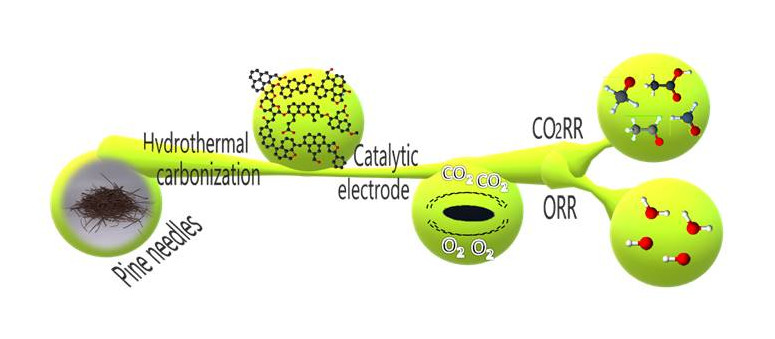
Hydrochar from Pine Needles as a Green Alternative for Catalytic Electrodes in Energy Applications
Authors: A. Marrocchi, E. Cerza, S. Chandrasekaran, E. Sgreccia, S. Kaciulis, L. Vaccaro, S. Syahputra, F. Vacandio, P. Knauth, M. L. Di Vona
Published on: Molecules. 2024, 29(14):3286. Doi: 10.3390/molecules29143286
Abstract
Hydrothermal carbonization (HTC) serves as a sustainable method to transform pine needle waste into nitrogen-doped (N-doped) hydrochars. The primary focus is on evaluating these hydrochars as catalytic electrodes for the oxygen reduction reaction (ORR) and carbon dioxide reduction reaction (CO2RR), which are pivotal processes with significant environmental implications. Hydrochars were synthesized by varying the parameters such as nitrogen loading, temperature, and residence time. These materials were then thoroughly characterized using diverse analytical techniques, including elemental analysis, density measurements, BET surface area analysis, and spectroscopies like Raman, FTIR, and XPS, along with optical and scanning electron microscopies. The subsequent electrochemical assessment involved preparing electrocatalytic inks by combining hydrochars with an anion exchange ionomer (AEI) to leverage their synergistic effects. To the best of our knowledge, there are no previous reports on catalytic electrodes that simultaneously incorporate both a hydrochar and AEI. Evaluation metrics such as current densities, onset and half-wave potentials, and Koutecky–Levich and Tafel plots provided insights into their electrocatalytic performances. Notably, hydrochars synthesized at 230 °C exhibited an onset potential of 0.92 V vs. RHE, marking the highest reported value for a hydrochar. They also facilitated the exchange of four electrons at 0.26 V vs. RHE in the ORR. Additionally, the CO2RR yielded valuable C2 products like acetaldehyde and acetate. These findings highlight the remarkable electrocatalytic activity of the optimized hydrochars, which could be attributed, at least in part, to their optimal porosity.
The Team