Amphoteric ionomers are a particular class of polyelectrolytes containing both acidic and basic groups in their structure. Typically, amphoteric polymers bear fixed positive and negative charges formed by weak basic and acidic moieties.
Among synthetic polyelectrolytes, amphoteric polymers are close to biological macromolecules. They may provide useful analogues of proteins and are important to model some properties and functions of biopolymers. A very attractive feature of amphoteric ion-exchange polymers is the possibility to control properties related to the ionic charge by changing the external pH. This allows designing smart stimulus-responsive soft materials with controlled property dependence. The consequent activity as anti-fouling materials, preventing adsorption of organic and biological macromolecules on the surface, is another important advantage of amphoteric polyelectrolytes. They are also relevant in separation technologies and drug delivery and in electrochemical energy technologies, such as polymer electrolyte membrane fuel cells and redox flow batteries. It was also reported that polymers with weak acid or basic groups can activate water splitting in bipolar membranes, considering that water splitting strongly depends on the interface characteristics. Different polyelectrolytes were used for this aim: anion exchange membranes with secondary amine or tertiary ammonium groups or proton exchange polymers with weak acidic groups.
In the LIME lab, we synthetized amphoteric ionomers by innovative synthesis methods, introducing sulfonic acid and sulfonamide groups into poly(ether ether ketone) (SAM-PEEK) or by non-hydrolytic sol-gel chemistry. In the latter case, they include a silica-based inorganic side chain with grafted functional groups, which allows developing materials with controlled microporosity and pH-sensitivity. The pH-dependent variations of various properties, such as hydration and ionic conductivity of acidic, basic and zwitterionic forms, gives the opportunity to tune and optimize these properties by an external stimulus.
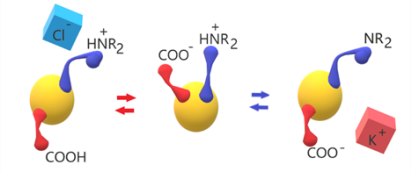
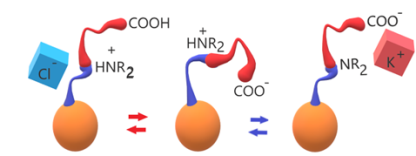
Polysulfone (PSU) was functionalized in order to produce two model cases of amphoteric ionomers: 1) acid and basic groups both in the same flexible side chain, made by an amino acid, 2) acid and basic groups separately tethered on the backbone. Hydration and ionic conductivity are dependent on the type and amount of ionic groups and are pH-dependent.